mRNA Vaccine Technology Being Studied for Head & Neck Cancer
Moffitt now has two cancer vaccine trials open in the Department of Head and Neck-Endocrine oncology. In one, physician scientists are currently studying a vaccine that uses the same technology as the COVID-19 vaccine for head and neck cancer.
While there currently are two approved immunotherapy treatments for head and neck cancers, only about 20% of patients respond. Vaccine trials like these will hopefully open the opportunity to satisfy a critical unmet need in this patient population.
Moffitt Cancer Center clinical trial number 21026 is a study of the Therapeutic Vaccine ISA101/ISA101b. This vaccine trial is specifically for patients with human papilloma virus (HPV)-associated head/neck cancers.
A large number of head and neck cancers are caused by an uncontrolled, persistent infection with high risk HPV. ISA101/ISA101b is a novel therapeutic synthetic long peptide (SLP) vaccine targeting HPV16. ISA101b Vaccine is being developed and has already shown efficacy in patients with high-grade premalignant vulvar lesions caused by HPV with only minor toxicity.
ISA101 consists of 12 synthetic long peptides (25 to 35 amino acids long) derived from the E6 and E7 oncogenic proteins of the HPV 16 virus, a strain responsible for more than 85% of HPV-positive head and neck cancers. In September 2021, ISA101b received a fast track designation from the FDA for the treatment of patients with recurrent and metastatic HPV16–positive oropharyngeal cancer.
"This is really remarkable because the vaccine is designed specifically to targets unique to the cancer that are not found on normal cells," explains Kedar Kirtane, MD, a medical oncologist in Moffitt’s Head and Neck Oncology Program.
The other vaccine trial -- Moffitt Cancer Center clinical trial number 21220 -- harnesses mRNA technology and is meant for patients whose cancers are not associated with HPV. The mRNA vaccine is truly personalized to an individual patient’s cancer with the hopes of stimulating the immune system in a really specific and unique way.
The study will assess the immunogenicity of mRNA-4157, an individualized, therapeutic personalized cancer vaccine targeting 20 tumor-associated antigens (TAAs) that are specifically expressed by the patient's cancer cells, with potential immunostimulatory and antineoplastic activities.
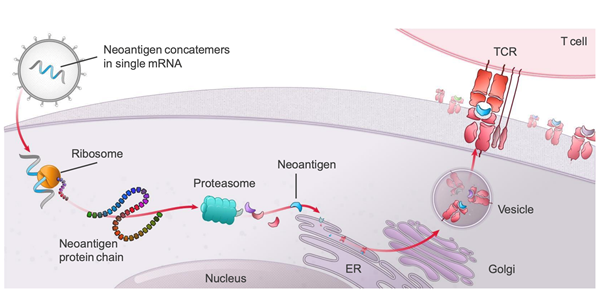
 PCV mRNA-4157 elicits T cell immune responses
PCV mRNA-4157 elicits T cell immune responses
"This is a much needed study because this group of patients tends to have a worse prognosis compared to patients whose cancers are related to HPV," Dr. Kirtane says.
"We are fortunate at Moffitt to have these trials and help push the needle of science forward for patients in our care."
To refer a patient to Moffitt Cancer Center for a trial eligibility consultation, call 1-888-663-3488, complete the online form or contact a physician liaison for assistance.
