Moffitt Treats its First Commercial TIL Therapy Patient Since FDA Approval
Moffitt Cancer Center has treated its first commercial patient with tumor-infiltrating lymphocyte (TIL) therapy. The one-time T-cell therapy infusion uses a patient’s own immune cells to fight their cancer and is the first FDA-approved cell therapy for solid tumors.
Bill Hoffman, 69, is the first patient at Moffitt to be given the therapy, known as lifileucel, since it was approved for commercial use by the FDA in February 2024.
In January 2021, Hoffman noticed two small dime-sized lumps on the left side of his neck. Doctors told him it was melanoma, and he had 25 lymph nodes removed. Five of those came back positive for melanoma.
“That’s where the journey really starts to get serious,” Hoffman said.
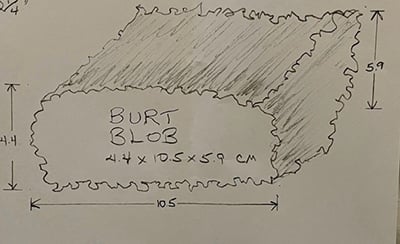
Hoffman drew this picture of his tumor after a scan and named it "Burt."
Hoffman was placed on immunotherapy infusions for 12 months. A CT scan in October 2023 showed that a tumorous mass, which he named “Burt,” had formed and his doctors were out of options.
That’s when Hoffman decided to come to Moffitt, where he learned of groundbreaking work being done on a new therapy for patients with his specific diagnosis.
“The timing couldn’t have been better because this is an aggressive cancer and at times I felt I could feel the cancer growing,” Hoffman said. “This was my best shot.”
TIL therapy takes advantage of the immune system’s ability to seek out and fight cancer cells. Melanoma tumors are surgically removed and then sent to a manufacturing facility where the immune cells, known as tumor-infiltrating lymphocytes, are removed and grown to billions in number. To make room for the new army of immune cells, patients receive chemotherapy to temporarily deplete their immune system before receiving the TIL infusion. The entire process takes about a month and a half from surgery to infusion.
Although the first TIL therapy for advanced melanoma was just approved by the FDA in 2024, the National Cancer Institute has been studying the therapy since the 1980s. After Moffitt researchers visited the institute to learn the protocol in 2009, Moffitt became the first cancer center outside of the institute to offer TIL to melanoma patients. During the clinical trial period for lifileucel, Moffitt was one of only five cancer centers nationwide that could manufacture a patient’s TIL cells, thanks to its multimillion-dollar Cell Therapies Core.
In total, Moffitt has opened eight TIL trials, treating about 100 patients. Led by Moffitt cutaneous surgical oncologist Amod Sarnaik, MD, a trial for patients whose advanced melanoma failed to respond to other treatments reported a 38% response rate to TIL, promising results for patients who have run out of treatment options.
Now that the first TIL product is approved, Moffitt experts are exploring ways to optimize the treatment. More trials are being conducted to find ways to cut down on manufacture time and create a better product with a higher success rate.

“We are not going to stop here. We aren’t going to be satisfied with what we have now. We are greedy in terms of wanting to get better responses for patients,” Sarnaik said.
On March 26, 2024, Hoffman underwent a tumor harvest where portions of “Burt” were removed and sent to a bioengineering facility where the commercial TIL product is now made. He recovered quickly, and then the waiting began.
“We asked them to tell us when Bill’s ‘friends’ were here,’” said Hoffman’s wife, Lorri, referring to the bioengineered cells.
On May 3, the Hoffmans learned that the expanded cells had arrived back at Moffitt, and that he was to be readmitted the next week. After five days of intense chemotherapy, he received the TIL infusion on May 16.
“I have confidence with this plan,” Bill Hoffman said before he was readmitted. “This is the future of treatment for metastatic melanoma, and Lorri has kept us organized. She has three-ring binders of information that bring us peace of mind and keep us from getting overwhelmed. It’s all critical information and easily accessible, which helps us compartmentalize the whole journey.”
Sarnaik says there is reason to be optimistic. Responses have been durable for patients, lasting well over two years.
“This breakthrough is the next generation of cellular immunotherapy and provides a much-needed option for metastatic melanoma patients,” he said. “TIL therapy is the most reliable option after traditional standard-of-care therapies have failed.”