20MA009 CAR-T: D99/CLEC12A AND gated Bispecific CAR-T for the Treatment of AML
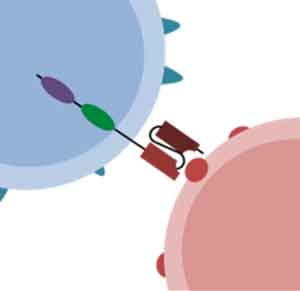
CD99 and CLEC12A are both tumor associated antigens over-expressed on AML blasts and leukemic stem cells but have low or absent expression on normal hematopoietic stem cells making them ideal targets for therapy. CD99/CLEC12A bispecific CAR-T constructs were designed using an AND gating strategy. The AND gated CARs use a split costimulatory and activation domains with CD28/41BB sequences on one CAR construct and CD3ξ on the other CAR construct. Therefore, target recognition and activation of both antigen binding domains is necessary to induce a cytotoxic effect. This ensures better specificity and lower off-target cytotoxicity than a traditional mono CAR-T. The Bispecific CARs had a favorable cytokine profile, demonstrated superior killing in-vivo as compared to CLEC12A mono CARs, and one CAR construct led to 100% survival at 21 days in a mouse model.
COMMERCIAL OPPORTUNITY
- Acute myeloid leukemia (AML) is characterized by excessive clonal proliferation of myeloid cells. Treatment options for AML include chemotherapy and allogeneic hematopoietic stem cell transplant (alloHSCT) for AML patients at high risk of relapse. However, the success rate for alloHSCT is limited with patients suffering from complications such as graft versus host disease. Therefore, an unmet need remains for the development of new therapies for AML.
- The marketplace is attractive for autologous CAR-T cell therapies, as Novartis received approval for Kymriah, its anti-CD19 CAR-T therapy for pediatric B-cell ALL with an ORR of 82.5%. Although the list price for Kymriah is $475,000 for a one-time treatment, Novartis has said only those patients who respond by the end of the first month will need to pay. Gilead’s Yescarta, an anti-CD19 CAR-T, was approved for large B-cell lymphoma and is listed at $375,000. Additional CARs have been approved including Gilead’s Tecartus, an anti-CD19 CAR-T for mantle cell lymphoma, Bluebird and BMS’ Abecma, an anti-BCMA CAR-T, for multiple myeloma. In 2017, Gilead acquired Kite Pharma for $11.7B, and in 2018, Celgene acquired Juno Therapeutics for $9B. Juno is also developing a CD-19 CAR-T therapy. In 2020, Kymriah had annualized sales of $422M, and Yescarta had annualized sales of $592M. Also, CMS in 2018 set Medicare Part B reimbursements for CAR T-cell therapies at $500,000 for Kymriah and $400,000 for Yescarta in the outpatient setting.
TECHNOLOGY
Based on in vitro safety and functional results, five bi-specific CARs were selected for in vivo efficacy experiments. Mice were given the AML tumor cell line MV4-11 three days before CAR T cell administration. Tumor progression was evaluated by bioluminescent imaging. Bioluminescent images of each mouse at specificed days after tumor injection is shown with average tumor burden (average radiance) of mice treated with bispecific CAR T cells and controls. Data shown as mean.
PUBLICATION/PATENT
PCT application was filed in July 2020 for Dr. Marco Davila
Haskell Adler PhD MBA
Senior Licensing Manager
Haskell.Adler@Moffitt.org
(813) 745-6596

