20MA005: PERK, IRE1: Method of Enhancing Immunotherapy Using ER Stress Pathway Inhibitors
IRE1α and PERK inhibitors may alter the function of T cells and myeloid cells in tumors to make cancer immunotherapy more effective. Because sustained IRE1α -XBP1 signaling inhibits the anti-cancer capacity of intratumoral T cells by dampening their metabolic fitness, IRE1α may increase CAR-T and TIL tumor cell killing. IRE1a expression was found to be lower in CAR-T cells in patients who responded to therapy. PERK signaling increased in tumor-myeloid-derived suppressor cells (MDSC), and its deletion, transformed MDSC into myeloid cells that activated anti-tumor CD8+ T-cell immunity. As such, PERK inhibitors may potentiate checkpoint inhibitors, as well as CAR-T cell or TIL therapies against solid tumors in combination therapy regimens by stopping the suppression of T cells.
COMMERCIAL OPPORTUNITY
- The tumor microenvironment disrupts the protein-folding capacity of the endoplasmic reticulum (ER) in infiltrating immune cells, thereby provoking a state of pathological “ER stress” that promotes immune evasion by cancer cells. Studies have demonstrated that two major ER stress response pathways, (inositol-requiring enzyme 1) IRE1α-XBP13,4 and (PKR-like endoplasmic-reticulum (ER) kinase) PERK-Chop5, cripple anti-cancer immunity by altering the function of myeloid cells and T cells in tumors. It has been shown in the setting of immunotherapy-refractory ovarian cancer that maladaptive activation of IRE1α -XBP1 in intra-tumor T cells limits their anti-cancer activity by impairing mitochondrial respiration and metabolic fitness. In addition, sustained activation of PERK-Chop intrinsically thwarts the effector function of ovarian tumor-infiltrating T cells, which also correlated with an impaired mitochondrial metabolic activity and limited expansion of protective tissue-resident memory (TRM) CD8+ T cells
- There is room for improvement in the therapeutic efficacy of treatments such as CAR-T cells in solid tumors, checkpoint inhibitors and TILs. This current response rate for CAR-T therapy in solid tumors is about 9% as shown in a review of 22 solid tumor CAR-T studies up to June 1, 2018 compared to a response rate for pediatric B-cell ALL of 82.5%. And there are 1.6 million new cases of solid tumors in the US, with 90% of all cancer cases being solid tumors.
- Opdivo, an anti-PD1 antibody showed a 32% response rate in the CHECKMATE-037 trial for unresectable or metastatic melanoma, and Iovance Biotherapeutics has a Phase 2 study ongoing with a reported Objective Response Rate of 36% in heavily pretreated metastatic melanoma patients. These data show that there is a potential to make both checkpoint inhibitors and TIL therapy more effective
TECHNOLOGY
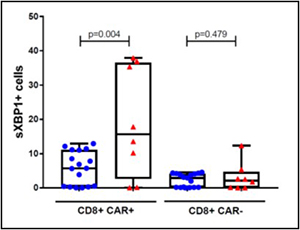
sXBP1 was evaluated by flow cytometry in viable peripheral blood CD3+CD8+ T cells collected day 7-12 after CAR T infusion, the most likely time of maximal concentration. The % of CD8+ CAR T cells expressing sXBP1 was higher in axicabtagene ciloleucel treated DLBCL non-responder patients than responder patients. Non-responder red triangle defined as those not achieving or not remaining in remission at day +90; responder blue circle defined as those achieving and not progressing prior to day +90.
PUBLICATION/PATENT
Provisional Patent Application filed on February 20, 2020 for Dr. Rodriguez
The Innovation Office
InnovationMarketing@Moffitt.org
(813) 745-6828

