13MA001 Med Device: Improved Enteral Feeding Tube and Retention Disc to Reduce Dislodgement Infection
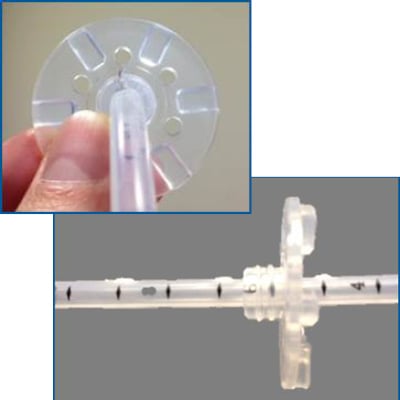
Current enteral feeding tubes deliver nutrition and medications directly into the stomach, duodenum, or jejunum and are commonly secured within the patient simply by friction with the retention disc. These tubes often become loose at the placement site resulting in gastric content leakage, infection, inadequate feeding and a need for tube replacement. Our improved enteral feeding tube has a modified retention disc system so that it interacts with and locks onto protrusions on the tube (see red arrows in image). This device may help to improve patient care and reduce hospital costs related to hospital acquired infection, trauma and wasted feeding tubes.
COMMERCIAL OPPORTUNITY
- Enteral feeding tubes are usually indicated for patients where normal oral intake of nutrients is insufficient (e.g., burn victims, cystic fibrosis) and/or unsafe due to anatomic or neurologic dysfunction (e.g., cancer, stroke, head trauma, GI dysfunction/obstruction, neuromuscular disease, etc.). An estimated 100,000-125,000 percutaneous gastrostomy (stomach) tubes are placed endoscopically each year in the US and about 96,000-120,000 gastrostomy and jejunostomy tubes are placed annually in Japan.
- With current enteral feeding tubes, the friction holding the external retention disc in place diminishes over time and the retention disc can slide out of place. This allows for greater “back and forth” motion of the tube and can cause gastric content leakage (peristomal leakage, 58%-78%), wound infection (5-47%), peritonitis (2.3%), accidental dislodgement (12.8%) and a need for tube replacement.
- Accidental gastrostomy dislodgement has been estimated to be a $23M problem in the US alone, often requiring repeated radiographic confirmation of placement and surgical intervention to replace the tube in a new site at a cost of $1200/dislodgement. Additionally, treatment of surgical site infections can cost upwards of $35,000 per event. Use of prophylactic antibiotics have reduced incidence of some infections, but may be resulting in an increased prevalence of MRSA infections around surgically placed feeding tubes.
- Our improved gastrostomy tube should reduce the need for extra securing methods that can decrease the lumen size of the tube and increase the risk of tube blockage, like current methods of keeping the retention disc in place, such as adding a plastic ring or clamp, tightening a silk suture around the neck of the disc, or tying a zip-tie around the disc.
TECHNOLOGY
This technology is a modified enteral feeding tube and retention disc that prevents the disc from slipping and the tube from dislodging. Our prototype tube has protrusions on its outer surface that pass through the retention disc when aligned with small openings on the disc. Rotating the retention disc engages the protrusions with the disc, resulting in a locked formation, securing the gastrostomy tube in place and reducing its ability to become loose. This lock/brake system is applicable for both balloon and mushroom gastrostomy tubes which require replacement at least every 3 to 6 months.
PUBLICATION/PATENT
- PCT patent application filed 12/29/2014 for Dr. Ghassan El Haddad
The Innovation Office
InnovationMarketing@Moffitt.org
(813) 745-6828

