18MB053 Novel BRD4/JAK2 Dual Inhibitor Cancer Therapeutics
Bromodomain-containing protein 4 (BRD4) is an epigenetic regulator that controls the expression of oncogenes such as c-Myc and Bcl-2. Inhibitors of BRD4 are being explored clinically for the treatment of solid and hematologic cancers. Our technology is a family of orally bioavailable small molecules that inhibit BRD4 with activities equivalent to that of the prototypical BRD4 inhibitor JQ1. In addition to inhibiting BRD4 at an IC50 of at least 4 nM, our compounds also potently inhibit the protein kinases JAK2 and FLT3, and they have IC50s for JAK2 that are comparable to those of the JAK2 inhibitors currently in the clinic and on the market. Several groups have shown synergistic promotion of apoptosis in cancer cells both in vitro and in vivo when the kinase and bromodomain proteins are inhibited together. Moffitt lead compounds show improved half lives compared to the parent molecule fedratinib, and show promising results in pre-clinical models of JAK2-driven myeloproliferative neoplasms (MPNs) resulting in about 99% tumor cell growth suppression.
COMMERCIAL OPPORTUNITY
- A synergistic increase in cell killing occurs when myeloproliferative neoplasm primary cells are treated in combination with BRD4 and JAK2 inhibitors, or when AML cell lines are treated with inhibitors of BRD4 and FLT3. JAK2-driven cells show no resistance to dual inhibitors, while the same cells develop rapid resistance to Ruxolitinib and Fedratinib. Ruxolitinib-resistant cells however remained highly sensitive to our lead dual inhibitor compounds, potentially overcoming a major clinical bottleneck in the effectiveness of currently available JAK2 inhibitors.
- The market for BRD4 inhibitors is attractive as evidenced by companies developing inhibitors. Constellation Pharmaceuticals has a pan-BRD inhibitor that is in a Phase 2 clinical trial in Ruxolitinib failures as monotherapy or in combination with Ruxolitinib. Also OncoEthix’s OTX015 successfully completed Phase I clinical studies, and Merck acquired OncoEthix in December 2014 for $110M upfront and $265M in future milestones. Both drug candidates suggest that inhibition of BRD4 is not deleterious to humans.
- The Moffitt dual BRD4/JAK2 inhibitors are built on a Fedratinib backbone, and Fedratinib was being developed by Impact Biomedicines for myelofibrosis and polycythemia vera. Once Fedratinib was taken off clinical hold by the FDA, Impact Biomedicines was acquired by Celgene for $1.1B upfront and up to up to $1.25 billion in contingent payments based on regulatory approval milestones for myelofibrosis. Ruxolotinib, Incyte’s and Novartis’ JAK1/JAK2 inhibitor, has already been approved for myelofibrosis, a rare bone cancer, as well as psoriasis, and rheumatoid arthritis, and had annual sales in 2018 of $1.4B.
TECHNOLOGY
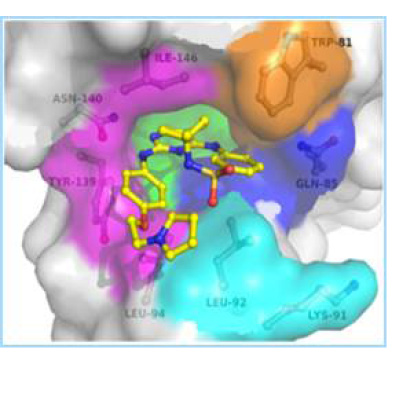
To develop dual acting BRD4-kinase inhibitors, a kinase inhibitor library was screened to identify compounds with binding potential for the acetyl-lysine binding site of BRD4. The JAK2/FLT3 inhibitors TG101348 (Fedratinib) and TG101209 were identified as moderately active inhibitors of BRD4. Using a structure based approach based on multiple X-ray co-crystal structures, a series of analogues that potently inhibit both BRD4 and JAK2 were designed and synthesized. Lead compounds are superior to Ruxolitinib and Fedratinib at inhibiting the neoplastic cell growth of primary cells from MPN patients. Lead compounds are orally bioavailable with favorable PK/PD properties in mice. Mice injected with BaF3-JAK2-V617F cells showed significant reduction of tumor growth upon 14 days of once daily oral administration of 50 mg/kg of our lead dual BRD4/JAK2, which led to 99.4% tumor cell growth suppression as measured by bioluminescence. This was superior to similar dosing of Fedratinib and Ruxolitinib, which demonstrat only 30% and 60% tumor cell growth reduction, respectively.
PUBLICATION/PATENT
Patent application filed September 7, 2018 for Drs. Lawrence, Lawrence, Schönbrunn and Reuther.
The Innovation Office
Innovationmarketing@Moffitt.org
(813) 745-6828

