21MB061 KRAS: Platform Technology: Dimeric IgA Therapeutic Antibody for Intra-Tumoral Targeting of Mutant KRAS
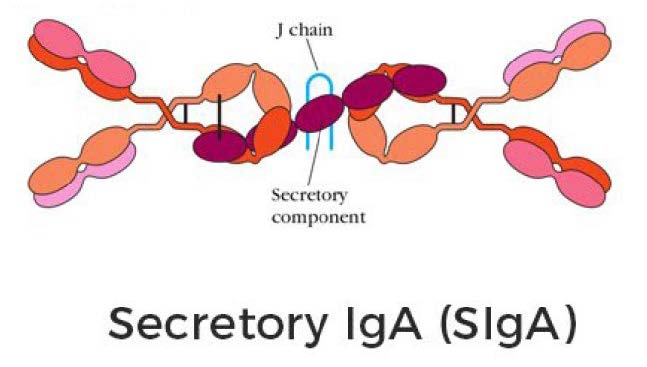
In order to target intracellular KRAS mutant proteins, an anti-KRAS G12D IgA antibody has been developed. Dimeric IgA antibodies linked with a J chain can enter cells by binding to the polymeric immunoglobulin receptor (pIgR). This transcytosis of IgA antibodies into the cell and then the subsequent secretion of the KRAS G12D bound to IgA has been shown to result in about a 90% reduction in tumor cell growth in vivo. The pIgR has been found to be broadly expressed on the cell surface of ovarian cancer cells. This platform technology represents a new way to inhibit intracellular targets where small molecule innovation may be slow to create the necessary therapeutics. New antibodies can be developed quickly with specificity and affinity for protein targets. This B-cell-related approach may be particularly effective in cancers such as ovarian cancers that are resistant to checkpoint inhibitors.
COMMERCIAL OPPORTUNITY
- Oncogenic mutations in KRAS lead to it being permanently bound by GTP (as opposed to GDP) rendering KRAS constitutively active. The mutated KRAS oncogene is found in approximately 90% of pancreatic adenocarcinomas, 40% of colorectal cancer, 30% of lung cancers, and generally in about 20-30% of all human cancers, with the G12D mutation being the most frequent at approximately 43%, representing nearly 50,000 US patients annually. These cancers are particularly difficult to treat—with a tendency to poor outcomes, due to an association between KRAS mutations and lack of response to EGFR tyrosine kinase inhibitors and chemotherapy.
- Amgen received second line approval in May 2021 for the first KRAS inhibitor, LUMAKRAS™ (sotorasib) for patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer. The drug was approved under accelerated approval based on overall response rate and duration of response and may require confirmatory trials. The drug is priced at $17,900 with consesus Wall Street estimates of sales over $800M; however, the G12C mutation is in only about 13% of NSCLC.
- Historically, by April 2021, the FDA had approved 100 antibody drugs and antibodies had twice the approval rate of small molecules. A study by Reichert looking at 569 antibodies from Phase 1 to approval between 2005 and 2014 showed an overall success rate of 22%. However, these were all IgM antibodies so intracellular IgA antibodies may have higher risk than IgM antibodies but still have a higher success rate than small molecules due to higher binding specificity that leads to fewer off target effects.
TECHNOLOGY
A dimeric IgA specifically targeting the G12D mutation in KRAS specifically prevents KRAS endosomal recycling, delays the tumor cell cycle and significantly delays the growth of G12D KRAS-mutant tumors in vivo. This technology can be applied to other intracellular mutated oncodrivers, using mutation-specific antibodies.
PUBLICATION/PATENT
Provisional patent application filed in September 2021 for Drs. Conejo-Garcia and Biswas.
The Innovation Office
InnovationMarketing@Moffitt.org
(813) 745-6828

