20MB062 Mutated Estrogen Receptor Neoantigen Peptide
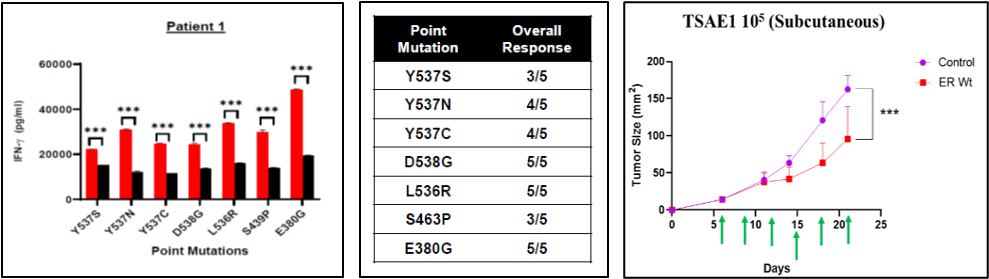
In patients that develop metastatic HR-positive breast cancer about 40-50% develop resistance to anti-estrogen therapy mediated by mutations in the ESR1 receptor. Moffitt investigators have developed neoantigens from seven mutated regions that result in CD4 Th1 responses that are stronger than immunogenic wild-type peptides that have been identified in ESR1. The results from patient #1 and the overall responses from five patients are shown below. Within the wild-type ESR1 four peptide epitopes were identified that routinely drive Th1 response. DC1 Dendritic cells were pulsed with these wild-type ESR1 peptides ex vivo and then were administered intratumorally twice a week for three weeks in tumor bearing Balb/C mice. Results show a significant delay in tumor growth using WT-DC1 vaccines (Fig). In addition, about a 12% of complete regression in these mice were observed. These results were quite exciting to us and suggest that mutations in ESR1 can lead to neoantigens driving a CD4 Th1 response that can also be used to potentially direct antibody responses and CD8 T cell cytotoxic responses.
COMMERCIAL OPPORTUNITY
- The American Cancer Society has estimated that 290,560 women will be diagnosed with breast cancer in 2022. About 80% of all breast cancers are HR-positive, and nearly 30% of women will develop metastatic disease. Furthermore, the prognosis for ER+ mBC is a median five-year survival rate of 27%, suggesting the need for new therapies.
- As of May 2018, there were three DC vaccines in clinical trials for the indications of Melanoma, Uveal Melanoma and Glioblastoma. Eight clinical trials are underway for the combination of DC vaccines and checkpoint blockade for various solid tumor malignancies.
- Three DC vaccine clinical trials are being pursued for the HER2/HER3 peptide vaccines for breast metastatic disease, brain cancer and leptomeningeal disease, at Moffitt and Roswell Park.
TECHNOLOGY
Neoantigen screening was done by obtaining human monocyte fractions from healthy donors and breast cancer patients; pulse said fractions with class II peptides; rapidly mature the fractions to a type-1 polarized dendritic cell (DC1) through the sequential addition of rhGM-CSF, rhIL-4, rhIFN-Ɣ and LPS; co-culture mature-peptide pulsed DC1’s with naïve T-cells wherein the T-cells are presented with peptides via MHC-II molecules and are polarized to a type-1 effector CD4+ cell through DC1 secretion of IL-12 creating primed CD4+ Th1 cells; re-stimulating the now primed CD4+ Th1 cells with immature dendritic cells presenting the matching class II peptide; obtaining supernatants from the iDC-CD4+ Th1 co-culture; and screening the supernatants using an immunoassay that measures T cell activity.
PUBLICATION/PATENT
PCT Patent application filed January 28, 2022, for Dr. Brian Czerniecki
The Innovation Office
InnovationMarketing@Moffitt.org
(813) 745-6828

