Behind the Microscope
Constance Kihm discovered her passion for dancing in her mid-60s. On a friend’s suggestion, she enrolled in a tap-dancing class, purchased her first pair of tap shoes for $75 and took a deep breath. She told herself she had better like this new, and what seemed at the time expensive, hobby. She ended up more than liking it. She fell in love with the group of women she danced with, the glittery costumes and performances. Those shoes became her favorite pair.
But in fall of 2018, at age 72, Kihm lay in a hospital bed, wondering if she would ever dance again.
Earlier that year, severe joint pain brought Kihm to an urgent care center near her home on Anna Maria Island, Florida. She was sent for a chest x-ray, and while doctors told her cancer was a possibility, it wasn’t likely. Kihm wasn’t convinced. “On both sides of my family, everyone died of cancer,” she said. “My dad died of testicular cancer at a young age and my mom died of lung cancer.”
Tests confirmed Kihm also had lung cancer. She was diagnosed with a rare genetic driver of non-small cell lung cancer in the ERBB2, or the HER2 gene, which causes less than 5% of all lung cancers, mostly in women who have no history of smoking. HER2, a protein called human epidermal growth factor receptor 2, promotes the aggressive growth of cancer cells.
Kihm underwent four rounds of chemotherapy but had no response to the treatment. She was then enrolled in a clinical trial. While the treatment was effective at the start, it came with debilitating side effects and eventually stopped working.
Still, Kihm was well enough to take a previously planned trip to South Africa. But while on the trip, every day found her more and more short of breath. Eventually, she was admitted to a hospital there.
“They took a chest x-ray and the cancer had come roaring back,” said Kihm. “I thought I was going to die in South Africa.”
After 10 days in the hospital, Kihm was medically transported back to the United States and began another round of chemotherapy. She was at her weakest point, struggling to breathe and dropping to under 100 pounds. The treatment worked, but doctors knew they needed to find a better, less-toxic long-term option.
The search was on to find Kihm a new clinical trial that would give her the best chance at survival.

Constance Kihm, cancer survivor
NEXT-GEN TESTING
When it comes to technology, we long for the next generation, the next big thing. We want the newest smart phone, the highest-resolution television, the most advanced car.
The completion of the Human Genome Project in 2003 was health care’s version of next generation technology. The project ushered in a new era of genomic analysis called next generation sequencing. Pathologists now could sequence millions of DNA molecules at the same time and investigate numerous different genes simultaneously, a process that in the past would have been too expensive and too time-consuming to be clinically feasible.
Since then, Moffitt Cancer Center has been continuously improving diagnostic molecular testing and using next generation sequencing to help provide the best patient care possible. It was this work that paved the way to a new treatment option for Kihm.
In 2018, Moffitt debuted a new investigative lab test, known as an assay, for solid tumors. This new test changed the game when it came to diagnosing and creating personalized treatment plans. Moffitt STAR is a 170-gene panel that not only looks for changes in tumor DNA, like other conventional next generation sequencing tests, but it also investigates changes in RNA, the shorter, single-stranded cousin of DNA. If DNA holds the body’s blueprint, RNA converts that information into the body’s building blocks.
“RNA testing opens up a whole new field and we are making discoveries that just haven’t been seen or investigated before,” said pathologist Theresa Boyle, MD, who helped build the new technology from the ground up. “When we make a diagnosis, we can be sure we’ve investigated the patient’s genes for any changes that lend themselves to known treatments. We also have enough information about the patient’s unique genetics to match them to a targeted therapy or to a Moffitt clinical trial aimed at those genetic findings.”
The FDA has approved certain treatments for tumors if a patient has a fusion in certain genes that can be identified. In the past, it was almost impossible for a lab to detect those fusions at the DNA level. Moreover, the fusions occur in such a small percentage of cases that it was hard to justify the time and cost of performing a separate lab test. Moffitt STAR can detect the fusions at the RNA level, and the results can give clear guidance to doctors on whether a patient would benefit from the treatment.
Moffitt STAR results not only can guide therapy, but also confirm diagnoses and, on a few occasions, have even overturned a wrong diagnosis. As the first institution to develop an integrated DNA- and RNA-based test, Moffitt has been a leader in this area and is helping other cancer centers develop a similar assay.
FASTER TURNAROUND
After looking at all the options, Kihm’s physician thought he found the best fit for her: an investigational drug that is designed specifically to turn off the HER2 gene. But to qualify for the clinical trial, Kihm had to have a positive test in the cancer tissue, not just in her blood, which was how she was originally diagnosed.
That’s where Moffitt STAR came in. The test can be run immediately after a tissue sample is collected because the test is done in-house at Moffitt.
This not only saves time but gives Moffitt physicians and researchers a better opportunity to analyze the data. On average, it takes about two weeks to get results and the team is working on making the turnaround time even faster.
Kihm’s physician told pathologists exactly what he was most interested in and how quickly he needed results. That fast-tracked Kihm’s case; the pathologist confirmed the result that was needed before taking the time to review the entire case with a fine-tooth comb.
The new approach also instills even more confidence for pathologists. Known as the “doctor’s doctor,” pathologists spend most of their time behind the curtain. They know the work they are doing behind the microscope is making a bigger impact on patients than ever before.
“I think it may be encouraging for patients to know when they’re seeing their doctor that a whole team of care providers are assisting the doctor to come up with best patient care based on their individual situation,” said Boyle.
That collaboration landed Kihm on the trial quickly, and more than four months later her tumor had shrunk 35%. She is considered stable, and says the few side effects like fatigue are very manageable.
“Of all the treatments, this is the best in terms of quality of life,” she said.
NEEDLE IN A HAYSTACK
Since the Moffitt STAR’s launch in February 2018 and July 2019 when this article was written, the test has been used in more than 800 cases. Moffitt has hired additional lab staff to run the test and has plans to develop automation and purchase a new sequencer with greater capacity to process more samples. Right now, the lab can process about 16 samples a week.
Moffitt pathologists are also rolling out another new assay this year that is similar to STAR. The myeloid action panel, or MAP, is a comprehensive 98-gene panel for blood and bone marrow cancers. While Moffitt STAR is used for solid tumors, MAP will be used for liquid ones and has the potential to be one of the best assays in the malignant hematology field.

Mohammad Hussaini, MD
“This is everything in one go, which is relatively unique,” said pathologist Mohammad Hussaini, MD. Before Moffitt MAP, pathologists had to do one expensive next generation sequencing test and then send out the sample for parallel testing to make sure they weren’t missing anything. “That’s added cost and time and now, with Moffitt MAP, we don’t have to do that anymore,” Hussaini said.
Identifying these mutations quickly and easily will help pathologists make a diagnosis, determine prognosis and help develop a more tailored treatment strategy. “Starting off on the right foot with the right test makes all the difference,” said Hussaini. “You can’t treat unless you have the proper diagnosis.”
All of the Moffitt STAR’s results go through Moffitt’s Personalized Medicine Group for further interpretation that is specific to each patient. The data is also kept for future research.
“As pathologists, we are stewards of the data and the samples, so it’s really important we get it to the researchers,” said Boyle. “It’s not just about patient care today, but the research to make things better in the future.”
With hundreds of cases run through Moffitt STAR, researchers have already started to see patterns emerge, specifically in advanced kidney cancer cases.
Epidermal growth factor receptor, or EGFR, is a protein that is amplified in cancer cells. It is most commonly found in lung cancer, and patients identified to have an EGFR mutation are eligible for certain targeted treatments. However, EGFR has never been significant in kidney cancer. All that changed once Moffitt STAR gave pathologists a look at RNA; they noticed a trend after running three kidney cancer samples.
“What we are finding is that it’s not at the DNA level that we are finding changes in EGFR in kidney cancer, but at the RNA level,” said genitourinary oncologist Brandon Manley, MD. “Only in kidney cancer cells is EGFR getting spliced at an abnormal rate way too often to just be a coincidence.”
Manley says this discovery is like finding a needle in a haystack. Now that they know what to look for, researchers can validate their finding and begin expanded studies. Manley has been recommended for a U.S. Department of Defense grant for additional research, and believes this could lead to important advancements in kidney cancer, the most lethal of all genitourinary cancers.

Brandon Manley, MD
“Given the fact that this is so unique to kidney cancer, the next question is: Is there some way that this helps the cancer survive, grow or spread?” Manley asked. “And more importantly, can we use this against the cancer? Can we use this to better monitor patients, screen patients, follow treatment outcomes, or even the Holy Grail: can we develop a specific drug to exploit this?”
DANCING AGAIN
Constance Kihm, almost two years since her diagnosis, said she finds it incredible how far cancer treatment has come in the last decades. Rather than the generic “one size fits all” chemotherapy treatment, doctors now have the ability to identify specific mutations that can dictate certain FDA-approved treatments or clinical trials. “They’ve come so far since my dad died of cancer in 1947,” said Kihm about the father she never got to know because cancer claimed his life.
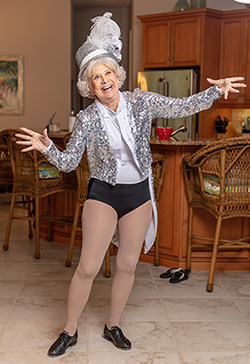
In July 2019, Kihm took her first tap class in eight months. "I was grinning from ear to ear," she said.
Kihm has also come far since landing in that hospital bed in South Africa. With her renewed quality of life, she is back to dancing. She started slow, taking a hula class which brought her back to the stage for her first performance in over a year.
In July, Kihm took yet another step forward—literally—lacing up those $75 tap shoes for her first tap class in eight months. “I was grinning from ear to ear,” she said. “I made it the entire class without having to sit down. It was the best feeling ever.”



